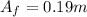Answer: 0.19 m
Explanation:- Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
![Rate=k[A]^x](https://img.qammunity.org/2020/formulas/physics/high-school/w7iriw6aoegvdiksrf8sti9boocc7ti08w.png) (1)
(1)
k= rate constant
x= order= 1
As the units of
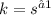 , the reaction follows first order kinetics.
, the reaction follows first order kinetics.
![Rate= 5.50* 10^(-3)* [0.550]^1=0.003ms^(-1)](https://img.qammunity.org/2020/formulas/physics/high-school/eir0ok14dfxmvs0gp1dixwxch91n6uvjwd.png)
Also rate is the change in concentration of reactants or products in a given time.
![Rate=(-d[A])/(dt)](https://img.qammunity.org/2020/formulas/physics/high-school/vyyffq1ysz5rcte2upcmwroaqy32f1ng3e.png) (2)
(2)
Equating (1) and (2)
![Rate=(-d[A])/(dt)=(-[A_f-A_i])/(dt)=k[A]](https://img.qammunity.org/2020/formulas/physics/high-school/mp3ofh27tt1wa5gshiq4e952vtwnbfi2td.png)
![0.003=-([A_f-0.550])/(120s)](https://img.qammunity.org/2020/formulas/physics/high-school/d90mk6p0ihco7865xjd6mmtvhvqzhpl2ix.png)