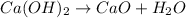Answer: The equations are provided below.
Step-by-step explanation:
Skeleton equations are defined as the equations which simply indicate the molecules that are involved in a chemical reaction. These equations are unbalanced equations.
Balanced equations are defined as the chemical equation in which number of individual atoms on the reactant side must be equal to the number of individual atoms on the product side.
Water decomposes in the direct current to form hydrogen and oxygen.
Skeleton Equation:
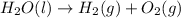
Balanced Equation:
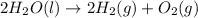
Mercury (II) oxide decomposes in heat to form mercury, oxygen.
Skeleton Equation:
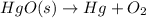
Balanced Equation:
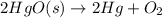
Calcium carbonate when heated forms calcium oxide and carbon dioxide.
Skeleton Equation:
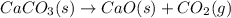
Balanced Equation:
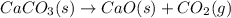
Group 2 hydroxides, when heated forms oxide and water vapor.
Skeleton Equation:
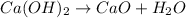
Balanced Equation: