Answer: Option (D) is the correct answer.
Step-by-step explanation:
A bond generated after transfer of an electron from one atom to another is known as an ionic bond. And, a bond generated by sharing of electron(s) between two atoms is known as a covalent bond.
Sodium is a metal with atomic number 11 and electronic distribution 2, 8, 1. On the other hand, chlorine is a non-metal with atomic number 17 and electronic distribution 2, 8, 7.
So, in order to attain stability sodium will lose one electron and develop a positive charge as
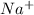 . Whereas chlorine at the same time will take up one electron from sodium atom and acquire a negative charge as
. Whereas chlorine at the same time will take up one electron from sodium atom and acquire a negative charge as
 .
.
Thus, we can conclude that the statement a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.