Answer:
Explanation:
(A) We know that Molarity=
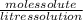
Since, moles solute= 0.0345 and litres solution =400mL=0.400l, thus
Molarity=
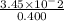
=
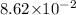
=
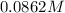
(B) Moles solute=Molarity×litres solution
=2.20×0.0350
=0.077 moles
(C) Using the formula,
Litres solution=
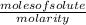
=
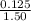
=0.0833L=83.33mL