Answer:
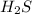
Step-by-step explanation:
Given the amount of heat absorbed and the amount of substance in moles, we may calculate the heat of vaporization. Heat of vaporization is defined as the amount of heat per 1 mole of substance required to evaporate that specific substance.
Based on the value of heat of vaporization, we will identify the substance. Firstly, let's calculate the heat of vaporization:
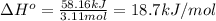
Secondly, let's use any table for heat of vaporization values for substances. We identify that the heat of vaporization of
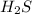 is 18.7 kJ/mol
is 18.7 kJ/mol