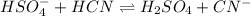Answer : The balanced reaction is,
Explanation :
According to the Brønsted-lowry concept, a substance is known as acid if it can donate a proton and as a base if it can accept a proton.
The given substance may be a molecule of an ion. The reaction of an acid with the base constitutes the transfer of a proton from acid to the base.
The balanced reaction will be,
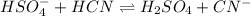
In this reaction,
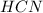 is an acid and is donating a proton to the base
is an acid and is donating a proton to the base
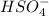 and both are the reactants.
and both are the reactants.
 is the conjugate acid of
is the conjugate acid of
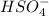 and
and
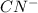 is the conjugate base of
is the conjugate base of
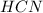 and
and
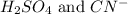 are the products.
are the products.
Hence, the balanced reaction is,