CH₄ + 2O₂ → CO₂ + 2H₂O
From the equation, we know that methane and carbon dioxide have the same number of moles.
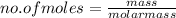
no. of moles of CO₂ produced = no. of moles of methane
= 4.5 × 10⁻³ ÷ (12 + 1×4)
= 2.8125 × 10⁻⁴
∴ mass of CO₂ = 2.8125 × 10⁻⁴ × (12 + 16×2)
= 12.375 × 10⁻³ g