Answer:The Ksp expression for the system
![K_(sp)=[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/mathematics/middle-school/s56gnrs0y8ewj88mg6jv3o3h9j4kofa4p9.png)
Explanation:
The
 od the substance that solubility product is defined as mathematical product of concentration of dissolved ions raised to the power equal to their stoichiometric coefficient in reaction.
od the substance that solubility product is defined as mathematical product of concentration of dissolved ions raised to the power equal to their stoichiometric coefficient in reaction.
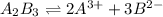
![K_(sp)=[A^(3+)]^2* [B^(2-)]^3](https://img.qammunity.org/2020/formulas/mathematics/middle-school/390iqhwxxepj0qn6msl5kpu5qoly7htopd.png)
For the given system:
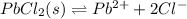
![K_(eq)=([PB^(2+)][Cl^-]^2)/([PbCl_2])](https://img.qammunity.org/2020/formulas/mathematics/middle-school/4138mpokst1y9a39dlciaj4hta5ujkbj1b.png)
![K_(eq)* [PbCl_2]=K_(sp)](https://img.qammunity.org/2020/formulas/mathematics/middle-school/gfspnzxn180n4byhfggxxcayyddkroci6i.png)
![K_(sp)=[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/mathematics/middle-school/s56gnrs0y8ewj88mg6jv3o3h9j4kofa4p9.png)
The Ksp expression for the system :
![K_(sp)=[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/mathematics/middle-school/s56gnrs0y8ewj88mg6jv3o3h9j4kofa4p9.png)