Answer :
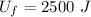
Explanation :
It is given that,
Heat that enters the system, Q = 1000 J
Work done by the system, W = -500 J
Initial internal energy
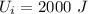
We know from first law of thermodynamics:
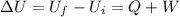
So, final internal energy will be :
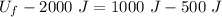
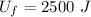
So, the final internal energy of the system is 2500 J.