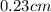Answer: The volume of aspirin is
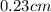
Step-by-step explanation:
To calculate mass of a substance, we use the equation:
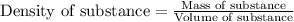
We are given:
Density of acetone =
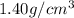
Mass of aspirin = 320 mg = 0.320 g (Conversion factor: 1 g = 1000 mg)
Putting values in above equation, we get:
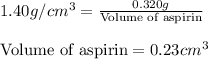
Hence, the volume of aspirin is