As we know that element will be represented as
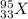
now here we know that
stormy number = 33
this represent the total number of protons in that atom
mass number = 95
this shows the total number of neutrons and protons inside an atom
so it is given as
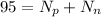
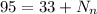
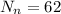
so number of neutrons are 62