Step-by-step explanation:
12)
a) Sodium + oxygen = ?
When sodium reacts oxygen its forms sodium oxide as a product.
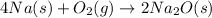
b) magnesium + fluorine = ?
When magnesium reacts with fluorine its forms magnesium fluoride as a product.
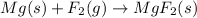
13)
a)
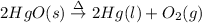
Decomposition of mercury(II) oxide on heating gives out mercury and oxygen gas.
b)
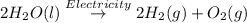
The electrolytic decomposition of water gives out hydrogen gas amd oxygen gas.