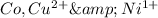Answer: The chemical symbols for three different atoms or atomic cations with 27 electrons are
Step-by-step explanation:
An atom is neutral specie with equal number of electrons and protons in it.
Atomic number = Number of protons = Number of electrons
Atomic number =- Number electrons = 27
The atom with 27 atomic number Cobalt that Co.
![[Co]=[Ar]3d^74s^2](https://img.qammunity.org/2020/formulas/chemistry/middle-school/fjg9zugay5bvfokc1rozxbqxq4npteeem4.png)
A cation is an ionic species which carry positive charge.When an atom looses an electron it forms cation.
Copper has atomic number of 29. When the copper atom looses 2 electrons it get converted into copper cation that is
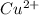 with 27 electrons in its ion.
with 27 electrons in its ion.
![[Cu]=[Ar]3d^(10)4s^1](https://img.qammunity.org/2020/formulas/chemistry/middle-school/qzkxytaqcfrx44s0zlwe5drf54oj1cao87.png)
![[Cu^(2+)]=[Ar]3d^(9)4s^0](https://img.qammunity.org/2020/formulas/chemistry/middle-school/lyevgleh98w0r12q8i1ol4e8h1h6nt8vqw.png)
Nickel has atomic number of 28.When the nickel atom looses 1 electron it get converted into nickel cation that is
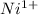 with 27 electrons in its ion.
with 27 electrons in its ion.
![[Ni]=[Ar]3d^(8)4s^2](https://img.qammunity.org/2020/formulas/chemistry/middle-school/93q0vnhw5r1qqb6r4t8uyavt5ycfp5kvv9.png)
![[Ni^(1+)]=[Ar]3d^(8)4s^1](https://img.qammunity.org/2020/formulas/chemistry/middle-school/9v54h0pjlo8yxzypmgpiei8la3bxbghp2e.png)
So,the chemical symbols for three different atoms or atomic cations with 27 electrons are