Answer: The correct answer is 15.2 g copper and 50.0 g copper and 11.2 g gold and 14.9 g gold.
Step-by-step explanation:
Density of an object is defined as the ratio of its mass and volume. The chemical equation representing density of an object is:
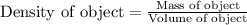
Density is considered as an intensive property. Intensive property is the property that does not depend on the amount of substance. It remains same.
The density of a particular substance remains the same.
For Example: Density of nickel will remain the same for 100 grams or 0.01 grams of nickel sample which is 8.908 g/mL
Hence, the correct answer is 15.2 g copper and 50.0 g copper and 11.2 g gold and 14.9 g gold.