Answer:
13.41 g/mL is the density of the metal.
Step-by-step explanation:
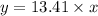
Slope intercept form of line:
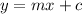
y = y, x= x m = 13.41 and c = 0
y= 13.41x + 0
Where y and x parameter as are the weights in grams and volume in milliliter respectively.
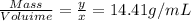
13.41 g/mL is the density of the metal.