Answer: They are often amines.
Explanation:
Covalent bases are lewis bases which can donate lone pair of electrons. Amines contain
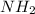 as the functional group and the lone pair on nitrogen can easily be shared.
as the functional group and the lone pair on nitrogen can easily be shared.
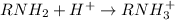
a) The bases which gives hydroxide ions in water are ionic bases as they are formed by complete transference of electrons. Example:
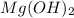
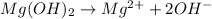
b) The substance which produce hydrogen ions are ionic acids. Example:

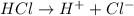
d) Covalent compounds are named differently than ionic compounds.