Answer: The lewis dots structure is given in the image attached. The molecular geometry of the given compound is trigonal pyramidal.
Step-by-step explanation:
We are given a molecule of
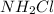
Lewis dot structure is defined as the structure which helps in the determination of number of bonded and non-bonded electrons. In this structure, the electrons are represented as dots. This structure is determined by counting the number of valence electrons around the atoms.
Valence electrons of nitrogen are 5, valence electrons of hydrogen are 1 and valence electrons of chlorine are 7.
Thus, the total number of valence electrons that are present in the molecule of
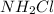 are:
are:
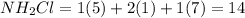
From the electron dot structure given below, it is clearly visible that number of bonding electrons are 6 and number of non-bonding electrons are 8.
Now, to determine the molecular geometry of
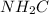 , we use the formula to calculate hybridization.
, we use the formula to calculate hybridization.
![\text{Number of electrons}=(1)/(2)[V+N-C+A]](https://img.qammunity.org/2020/formulas/chemistry/high-school/m0e43q2maagi835jtgnamjzz1rbba4ad2w.png)
where,
V = number of valence electrons present in central atom i.e. nitrogen = 5
N = number of monovalent atoms bonded to central atom = 3
C = charge of cation = 0
A = charge of anion = 0
![\text{Number of electrons}=(1)/(2)* [5+3]=4](https://img.qammunity.org/2020/formulas/chemistry/high-school/ett6nrhuqhfufgybix2jo70w3d6v0hee1h.png)
As, the number of electrons in
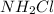 is coming out to be 4. So, the hybridization around the central metal atom will be
is coming out to be 4. So, the hybridization around the central metal atom will be
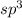 and the electronic geometry of the given molecule will be tetrahedral.
and the electronic geometry of the given molecule will be tetrahedral.
But, the atoms around nitrogen atom are three (1 chlorine and 2 hydrogen atoms), so the fourth position will be occupied by the lone pair present around nitrogen atom. Thus, the molecular geometry of this molecule will be trigonal pyramidal.
Thus, the lewis dots structure is given in the image attached. The molecular geometry of the given compound is trigonal pyramidal.