Answer: The correct answer is option (C).
Step-by-step explanation:
Pressure of the gas = 101.3 kilopascal
Volume of the gas = 495 mL = 0.495 L
Temperature of the gas = 25°C = 298 K(0°C = 273 K)
Value of gas constant in in kilopascals =8.314 kPa L\K mol
According to the ideal gas equation:'

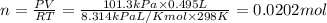 Moles of carbon-dioxide gas = 0.0202 moles
Moles of carbon-dioxide gas = 0.0202 moles
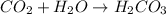 .
.
According to reaction 1 mole of
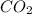 gas gives 1 moles
gas gives 1 moles
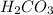 , then 0.0202 moles of
, then 0.0202 moles of
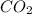 will produce :
will produce :
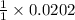 moles of
moles of
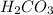 that 0.0202 moles.
that 0.0202 moles.
Mass of
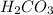 = Moles Molar mass of
= Moles Molar mass of
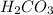
=
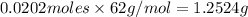
From the options given the closest value to our answer is of option(C).
Hence ,the correct answer is option C.