Explanation :
Voltaic cells are an electro chemical cell in which chemical reactions occurs to produce electrical energy. Its parts are :
(i) At anode oxidation occurs.
(ii) At cathode reduction occurs.
(iii) A salt bridge.
(iv) External circuit.
(v) Load.
Suppose a voltaic cell is made up of
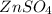 and
and
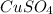 . Here zinc can lose electron more rapidly as that of copper. Oxidation of zinc occurs and is called as anode. So, this is a negative terminal.
. Here zinc can lose electron more rapidly as that of copper. Oxidation of zinc occurs and is called as anode. So, this is a negative terminal.
On the other hand, copper receives electron and hence become cathode. So, this is a positive terminal.
Hence, electrons flow form Zinc to copper i.e. from negative terminal to positive terminal.