Answer:
As per the given statement:
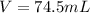
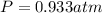
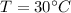
Using ideal gas law equation:
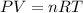
where
P represents the Pressure of a gas
V represents the Volume it occupies
R represents the Universal constant , usually given as:
R = 0.082 atm L/ mol k
First convert Celsius into Kelvin.
T = 273 + 30 = 303 K
Use conversion:
1 L = 1000 mL
V = 74.5 mL =
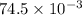 L
L
Now substitute the given values we have;
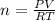
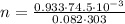
Simplify:
n = 0.00279757305
or
n ≈0.0028 moles.
Since carbon monoxide, CO has molar mass of 28.01 g/mol
then;
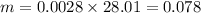 g
g
Therefore, 0.078 g of CO(g) are there in 74.5 mL of the gas at 0.933 atm and 30 degree C