Answer:
B) more dense; change to a liquid or solid
Step-by-step explanation:
When the pressure of a gas is increased, the average distance between the molecules decreases. In fact, if we assume that the temperature of the gas is kept constant, then we have
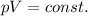
which means that the product between pressure and volume is constant: so, if the pressure increases, the volume decreases, and therefore the density of the gas increases (because density is inversely proportional to the volume,
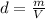 )
)
Moreover, at very high pressure, the gas can turns into a liquid and eventually into a solid: in fact, as the average distance between the molecules decreases more and more, at some point they becomes so close to each other that the intermolecular forces start to be relevant, turning the gas into a liquid.