Answer : The molarity of the new solution is, 4.069 M
Solution : Given,
Molarity of KOH solution = 4 M
Volume of KOH solution = 2.5 L
Volume of water added = 1.8 L
First we have to calculate the volume of new solution.
Volume of new solution = volume of KOH solution + volume of water added
Volume of new solution = 2.5 L + 1.8 L = 4.3 L
Now we have to calculate the molarity of the new solution.
Formula used :

where,
 = molarity of KOH solution
= molarity of KOH solution
 = molarity of new solution
= molarity of new solution
 = volume of KOH solution
= volume of KOH solution
 = volume of new solution
= volume of new solution
Now put all the given values in the above formula, we get the molarity of the new solution.
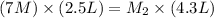
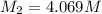
Therefore, the molarity of the new solution is, 4.069 M