Answer:
The correct answer option is B) 2.0 M.
Step-by-step explanation:
We are given the number of grams of NaOH (Sodium Chloride) which are dissolved in 750 milliliters of water and we are to find its molarity.
We know the formula of molarity:
Molarity = (mass * 1000) / (volume * molecular mass)
Volume = 750 ml = 750 cm

Molecular mass = 40
Mass = 60 grams
Substituting these values in the above formula:
Molarity =
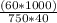 = 2.0 M
= 2.0 M