Answer:
2500 J
Step-by-step explanation:
The first law of thermodyniamics states that:
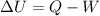
where
 is the change in internal energy
is the change in internal energy
Q is the heat absorbed by the system
W is the work done by the system
in this problem, Q=1000 J and W=500 J, so we have

Since the initial internal energy is
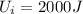 , we the final energy is given by:
, we the final energy is given by:
