15 gallons of 35% peroxide solution contains 0.35 × 15 = 5.25 gallons of hydrogen peroxide. The rest of the volume is made up of 15 - 5.25 = 9.75 gallons of water.
If we add
 gallons of water to the solution, the amount of hyrdrogen peroxide in the solution stays the same, but the volume of water increases to
gallons of water to the solution, the amount of hyrdrogen peroxide in the solution stays the same, but the volume of water increases to
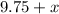 gallons, and the total volume increases to
gallons, and the total volume increases to
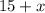 gallons.
gallons.
To get a concentration of 8% in this diluted solution, we add
 gallons of water such that
gallons of water such that
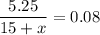
Solve for
 .
.
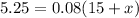
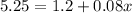
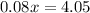
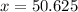
To get the desired concentration, one must add 50.625 gallons of water, and one will end up with a total volume of 65.625 gallons of solution.