Half life is the period of time it takes for one-half of the atoms of an original isotope to decay.
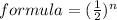
In this example we have:
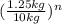 where n= the number of half lives.
where n= the number of half lives.
Thus:
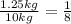
 From this equation n=3. The answer to your question is: 3 half lives have passed which gives the meteorite an age of 4.5*3= 13.5 billion years.
From this equation n=3. The answer to your question is: 3 half lives have passed which gives the meteorite an age of 4.5*3= 13.5 billion years.