Answer:
An increase in pressure
Step-by-step explanation:
The ideal gas law states that:
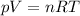
where
p is the gas pressure
V is the volume
n is the number of moles
R is the gas constant
T is the temperature of the gas
in the equation, n and R are constant. For a gas kept at constant volume, V is constant as well. Therefore, from the formula we see that if the temperature (T) is increase, the pressure (p) must increase as well.