- n = 3
- l = 0
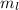 = 0
= 0
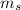 = 1/2 or -1/2
= 1/2 or -1/2
Step-by-step explanation
There are four quantum numbers in an electron that orbits the atom.
- n, the principal quantum number.
- l, the angular quantum number.
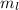 , the magnetic quantum number.
, the magnetic quantum number.
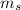 , the spin quantum number.
, the spin quantum number.
n is a positive integer. The value of n indicates the main shell of the electron. The electron in question is in the 3s orbital. As a result, n = 3.
l is a non-negative integer. The value of l indicates the type of subshell ("orbital") of the electron. The types of subshells possible depends on the main shell. For example, both s and p orbitals exist in the second main shell. However, only the s orbital exists in the first main shell. The value of l ranges from 0 to n - 1.
- l = 0 indicates an s orbital.
- l = 1 indicates a p orbital.
- l = 2 indicates a d orbital.
- l = 3 indicates an f orbital.
The electron in question is in an s orbital. As a result, l = 0.
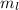 is an integer. The value of
is an integer. The value of
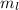 indicates the position of the electron within the subshell. The range of
indicates the position of the electron within the subshell. The range of
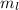 depends on the value of l.
depends on the value of l.
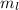 ranges from -l to l (that's -l, ..., -1, 0, 1, ... l). Accordingly, there are 2 l + 1 orbitals in a l subshell. l = 0 for this 3s electron. There's only one orbital in the 3s subshell. The only
ranges from -l to l (that's -l, ..., -1, 0, 1, ... l). Accordingly, there are 2 l + 1 orbitals in a l subshell. l = 0 for this 3s electron. There's only one orbital in the 3s subshell. The only
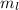 value possible for this electron is 0.
value possible for this electron is 0.
The value of
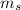 is either - 1/2 or 1/2. It indicates the position of an electron within a single orbital. The value of
is either - 1/2 or 1/2. It indicates the position of an electron within a single orbital. The value of
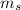 does not depend on that of n, l, or
does not depend on that of n, l, or
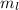 . However, by the Pauli Exclusion Principle, at least one of the four numbers must differ for two electrons in the same atom. In case all three of n, l, and
. However, by the Pauli Exclusion Principle, at least one of the four numbers must differ for two electrons in the same atom. In case all three of n, l, and
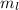 are the same, the two electrons must differ in
are the same, the two electrons must differ in
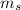 . However, this question asks only for the number of one single electron. Thus, giving either - 1/2 or 1/2 shall work.
. However, this question asks only for the number of one single electron. Thus, giving either - 1/2 or 1/2 shall work.
Reference
Vitz et. al, "5.8 Quantum Numbers (Electronic)", ChemPRIME (Moore et al.), Chemistry Libretexts. 27 Oct 2017.