Answer: The word equation for the given chemical equation is given below.
Step-by-step explanation:
Word equation is defined as the equation in which substances are written in word form and not in chemical formulas. The words "and" or "plus" means that one substance and another substance are both reactants or products.
For the given chemical equation:
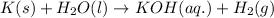
The word equation for this follows:
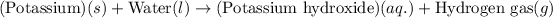
Here, 1 mole of potassium metal reacts with liquid water to produce 1 mole of potassium hydroxide and 1 mole of hydrogen gas.
Hence, the word equation for the given chemical equation is given above.