Answer : The number of drops it takes from a dropper to dispense 1.0 ml of ethanol is, 20 drops
Solution : Given,
Density of ethanol = 0.80 g/ml
Mass of ethanol = 0.60 g
First we have to calculate the volume of ethanol.
Formula used :

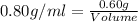
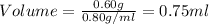
The volume of ethanol is, 0.75 ml
Now we have to calculate the number of drops it takes from a dropper to dispense 1 ml of ethanol.
As, the number of drops in 0.75 ml of ethanol = 15
So, the number of drops in 1.0 ml of ethanol =
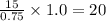
Therefore, the number of drops it takes from a dropper to dispense 1.0 ml of ethanol is, 20 drops