Step-by-step explanation:
When an atom gains electrons then it tends to obtain negative charge whereas when an atom loses electrons then it tends to gain a positive charge.
Atomic number of phosphorous is 15 and it need 3 more electrons in order to complete its octet. Therefore, by gaining electrons it will result in the formation of
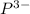 ion.
ion.
Thus, we can conclude that phosphorous can form an ion called phosphide, which has the formula
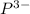 . This ion contains 18 electrons.
. This ion contains 18 electrons.