Answer: The beta-particle is being released in the reaction and the nucleus is changing from to nitrogen.
Step-by-step explanation:
Carbon-14 undergoes a radioactive decay by the process of beta-minus decay.
In beta-minus decay, a neutron gets converted to a proton and an electron.
The equation for the beta-minus decay of carbon-14 follows the reaction:
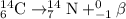
In this reaction beta-particle is being released carrying -1 charge. Another name for this particle is known as electron.
In this decay process, the nucleus is changing from carbon to nitrogen. The property of the nucleus is changing completely as number of protons is getting increased.