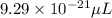Answer: The volume of vanadium atoms in microliters will be
Step-by-step explanation:
We are given volume of a vanadium atom which is
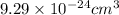 and we need to convert it into microliters.
and we need to convert it into microliters.
For this, we will use the conversion factor:
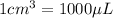
Converting this quantity into microliters by multiplying it with 1000, we get:
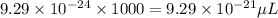
Hence, the volume of vanadium atoms in microliters will be