Answer: The mole ratio of H : O in ammonium nitrate is 4 : 3.
Step-by-step explanation:
We are given a compound named ammonium nitrate having formula
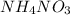
There are 3 elements in this compound which are nitrogen, hydrogen and oxygen.
To calculate the mole ratio, we write the ratio of their subscripts. For this compound, it is:
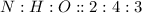
The mole ratio of H and O for this compound is 4 : 3.