Answer: decreasing pressure
Explanation: Solubility of a gas is governed by Henry's law.
According to Henry's law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas in equilibrium with the liquid at that temperature.
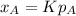
where,
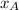 = mole fraction of gas in the solution
= mole fraction of gas in the solution
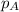 = partial pressure of the gas above solution
= partial pressure of the gas above solution
K= constant
The solubility of gas increases with decrease in temperature.
Thus the solubility of gaseous solute will decrease when the pressure is decreased.