Step-by-step explanation:
Both alcoholic group and carboxylic group are polar in nature.
This is because in carboxylic groups are both hydrogen bond acceptor and hydrogen bond donor. Also there is presence of two electronegative oxygen atoms.
Whereas in alcoholic groups also there is hydrogen bonding and there is one electronegative oxygen atom present.
Hence, it makes carboxylic group more polar.
Thus, we can conclude that
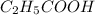 and
and
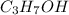 both are polar in nature.
both are polar in nature.