- Hydrogen bonds (strongest).
- Dipole-dipole interactions (weaker than hydrogen bonds).
Step-by-step explanation
There are three major types of intermolecular attractions:
- Hydrogen bonds,
- Dipole-dipole interactions, and
- London dispersion forces.
London dispersion forces exist between all molecules. It is the weakest among the three types of forces.
Dipole-dipole interactions exist between polar molecules. It is stronger than London dispersion forces.
Hydrogen bonds are attractions between
- H atoms directly bonded to atoms of N, O, or F; and
- Lone pairs of electrons on other molecules.
Hydrogen bonds is the strongest among the three types of forces.
Consider the structure of an ammonia
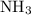 molecule.
molecule.
- All three H atoms are bonded to an N atom.
- There is one lone pair of electrons on the central N atom.
- N-H bonds are highly polar.
- The shape of the
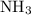 molecule is pyramidal. As a result, dipoles from the three N-H bond remain not balanced. The molecule is overall polar.
molecule is pyramidal. As a result, dipoles from the three N-H bond remain not balanced. The molecule is overall polar.
As a result,
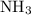 molecules will form both hydrogen bonds and dipole-dipole interactions between them. There's also going to be London dispersion forces. Nonetheless, hydrogen bonds and dipole-dipole interactions will contribute to the majority of attractions between the molecules.
molecules will form both hydrogen bonds and dipole-dipole interactions between them. There's also going to be London dispersion forces. Nonetheless, hydrogen bonds and dipole-dipole interactions will contribute to the majority of attractions between the molecules.