Answer :
Isoelectronic means that the element which have the same number of electrons.
The element is oxygen. The number of electrons is 8. The number of electrons in
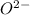 = 8 + 2 = 10
= 8 + 2 = 10
The element is sulfur. The number of electrons is 16. The number of electrons in
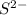 = 16 + 2 = 18
= 16 + 2 = 18
The element is selenium. The number of electrons is 34. The number of electrons in
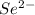 = 34 + 2 = 36
= 34 + 2 = 36
The element is tellurium. The number of electrons is 52. The number of electrons in
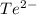 = 52 + 2 = 54
= 52 + 2 = 54
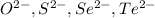 it is not an isoelectronic series because number of electrons are different.
it is not an isoelectronic series because number of electrons are different.
The element is sodium. The number of electrons is 11. The number of electrons in
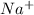 = 11 - 1 = 10
= 11 - 1 = 10
The element is potassium. The number of electrons is 19. The number of electrons in
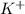 = 19 - 1 = 18
= 19 - 1 = 18
The element is rubidium. The number of electrons is 37. The number of electrons in
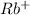 = 37 - 1 = 36
= 37 - 1 = 36
The element is cesium. The number of electrons is 55. The number of electrons in
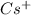 = 55 - 1 = 56
= 55 - 1 = 56
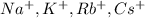 it is not an isoelectronic series because number of electrons are different.
it is not an isoelectronic series because number of electrons are different.
The element is nitrogen. The number of electrons is 7. The number of electrons in
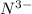 = 7 + 3 = 10
= 7 + 3 = 10
The element is phosphorous. The number of electrons is 15. The number of electrons in
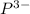 = 15 + 3 = 18
= 15 + 3 = 18
The element is arsenic. The number of electrons is 33. The number of electrons in
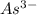 = 33 + 3 = 36
= 33 + 3 = 36
The element is antimony. The number of electrons is 51. The number of electrons in
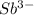 = 51 + 3 = 54
= 51 + 3 = 54
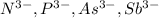 it is not an isoelectronic series because number of electrons are different.
it is not an isoelectronic series because number of electrons are different.
The element is fluorine. The number of electrons is 9. The number of electrons in
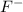 = 9 + 1 = 10
= 9 + 1 = 10
The element is chlorine. The number of electrons is 17. The number of electrons in
 = 17 + 1 = 18
= 17 + 1 = 18
The element is bromine. The number of electrons is 35. The number of electrons in
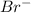 = 35 + 1 = 36
= 35 + 1 = 36
The element is iodine. The number of electrons is 53. The number of electrons in
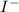 = 53 + 1 = 54
= 53 + 1 = 54
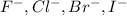 it is not an isoelectronic series because number of electrons are different.
it is not an isoelectronic series because number of electrons are different.
The element is silver. The number of electrons is 47.
The element is cadmium. The number of electrons is 48. The number of electrons in
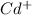 = 48 - 1 = 47
= 48 - 1 = 47
The element is tin. The number of electrons is 50. The number of electrons in
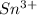 = 50 - 3 = 47
= 50 - 3 = 47
The element is antimony. The number of electrons is 51. The number of electrons in
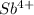 = 51 - 4 = 47
= 51 - 4 = 47
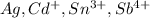 it is an isoelectronic series because number of electrons are same.
it is an isoelectronic series because number of electrons are same.
The element is fluorine. The number of electrons is 9. The number of electrons in
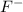 = 9 + 1 = 10
= 9 + 1 = 10
The element is neon. The number of electrons is 10.
The element is sodium. The number of electrons is 11. The number of electrons in
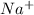 = 11 - 1 = 10
= 11 - 1 = 10
The element is magnesium. The number of electrons is 12. The number of electrons in
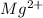 = 12 - 2 = 10
= 12 - 2 = 10
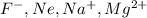 it is an isoelectronic series because number of electrons are same.
it is an isoelectronic series because number of electrons are same.
The element is sulfur. The number of electrons is 16.
The number of electrons in
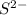 = 16 + 2 = 18
= 16 + 2 = 18
The number of electrons in
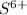 = 16 - 6 = 10
= 16 - 6 = 10
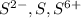 it is not an isoelectronic series because number of electrons are different.
it is not an isoelectronic series because number of electrons are different.