Explanation: We use a different element in the chemical equation.
We explain by a example
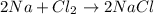
In this equation, we use the two elements Na and Cl.
Now, we count the number of atoms present on one side 2 atoms of Na and 2 atoms of Cl then the total numbers will be 4 .
But , we use a molecular formula then we count the number of each element of the molecular formula.