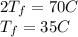Answer:
35 C
Step-by-step explanation:
At thermal equilibrium, the heat given by the hotter mass of water must be equal to the heat absorbed by the colder water (because energy cannot be destroyed nor created). In formula:
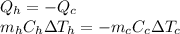
where:
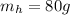 is the mass of the hot water
is the mass of the hot water
Ch is the specific heat of the hot water
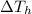 is the variation in temperature of the hot water
is the variation in temperature of the hot water
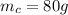 is the mass of the cold water
is the mass of the cold water
Cc is the specific heat of the cold water
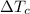 is the variation in temperature of the c water
is the variation in temperature of the c water
We notice that:
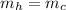 since the two masses are equal, and
since the two masses are equal, and
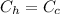 because the substance is the same (water)
because the substance is the same (water)
So, the above equation just simplifies as
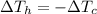
which can be rewritten as:
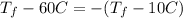
where T_f is the temperature of both masses of water at equilibrium. By solving the equation, we find