Answer:
The correct answer is 0.89 L
Step-by-step explanation:
To find the number of moles,
Moles = n=m/M.
n = number of moles, m is the mass in grams, and M is the molecular weight in grams per mole.
Given ,
Mass= 1.60g
Molar mass of Argon = 39.94g
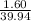
⇔ 0.04 mole
Now, 1 mole of gas takes up 22.4 L of volume.
0.04 mole = 22.4 × 0.04 =0.89 L
Thus, the volume occupied by the Argon is 0.89L