Answer: D. Catalyst.
Step-by-step explanation:
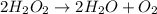
A. Reactants of a chemical reaction is defined as the substances which take part in a chemical reaction and undergoes a chemical change. Reactants are written on left side of the arrow.
B. Products are defined as the substance which are formed at the end of the chemical reaction.Products are written on the right side of the arrow.
C. Precipitate is a product formed which is formed as a solid and is not soluble in water.
D. Catalyst is the substance which does not participate in the reaction but increases the rate of the reaction and results in the early formation of product. It does not get consumed in the reaction and thus does not appear in the reaction.