mass number of uranium 238 is sum of number of proton and neutrons in it
While atomic number is just number of protons
So total number of protons in it = 92
total number of neutrons in it = 238 - 92 = 146
mass of proton = 1.0073 amu
mass of neutron = 1.0087 amu
now for the calculation of mass of nuclei is given as
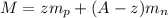
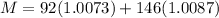
so option B is correct