Answer : The solubility of a gas in water at 1 atm pressure is, 0.4436 g/L
Solution : Given,
Solubility of a gas in water = 1.22 g/L (at 2.75 atm pressure)
At pressure = 1 atm, Solubility of a gas = ?
Now we have to calculate the solubility of a gas.
At 2.75 atm pressure, the solubility of a gas in water = 1.22 g/L
At 1 atm pressure, the solubility of a gas in water =
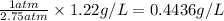
Therefore, the solubility of a gas in water at 1 atm pressure is, 0.4436 g/L