Answer: The number of moles of sodium chloride are 0.40 moles
Step-by-step explanation:
We are given:
Number of formula units =
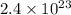
According to mole concept:
 number of formula units are contained in 1 mole of a substance
number of formula units are contained in 1 mole of a substance
So,
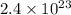 number of formula units will be contained in
number of formula units will be contained in
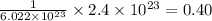 moles of sodium chloride
moles of sodium chloride
Hence, the number of moles of sodium chloride are 0.40 moles