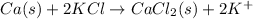Answer: Option (A) is the correct answer.
Step-by-step explanation:
When calcium reacts with potassium chloride then it results in the formation of calcium oxide and potassium ions.
The given equation is as follows.
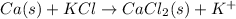
Number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
To balance the given equation, multiply KCl by 2 on the reactant side and multiply potassium ions by 2 on product side. Therefore, balanced chemical equation will be as follows.