Answer:- It indicates that there are 3 separate oxygen molecules in the reaction.
Explanations:-
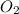 is oxygen molecule in which two O atoms are bonded together with a double bond(O=O). The numbers written in front of the chemical formulas in an equation are the coefficients and they are used to balance the equations.
is oxygen molecule in which two O atoms are bonded together with a double bond(O=O). The numbers written in front of the chemical formulas in an equation are the coefficients and they are used to balance the equations.
The balanced equation has 3 molecules of
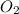 and all these molecules are separate from each other as the bonds are between two oxygen atoms of a molecule but not between the oxygens of two molecules.
and all these molecules are separate from each other as the bonds are between two oxygen atoms of a molecule but not between the oxygens of two molecules.
Of course there are 6 oxygen atoms in total but they all are not bonded together as a single molecule.
So, the right choice is D) It indicates that there are 3 separate oxygen molecules in the reaction.