Answer:
none of the above
Step-by-step explanation:
The actual answer is '91 protons'. In fact, the beta decay of the thorium-234 is the following:
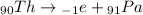
where inside the nucleus of Thorium (90 protons), a neutron turns into an electron (the beta particle) + a proton. Therefore, the resulting nucleus (which is Protoactinium) has a total of 90+1 = 91 protons.
So, the correct answer would be '91 protons'.