Answer: The temperature of the aluminium pan was decreased by the 117.25 Kelvins.
Step-by-step explanation:
Energy given by the aluminium pan =Q= -5400 J
Negative sign indicates that energy is released by the aluminium pan.
Mass of the pan=m = 50 g = 0.05 kg(1000g = 1kg)
Change in temperature =

Specif heat of he aluminium = c = 921.096J/kg K
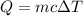
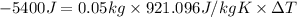
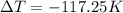
The negative value of change is temperature means that temperature of the aluminium pan was decreased by the 117.25 Kelvins.