Answer: 2.23
Step-by-step explanation:
The dissociation of acetic acid is as follows:
HCOOH(aq) ⇋ HCOO− (aq) + H+(aq)
The ICE table for the concentrations of ions is given below. From the table, the concentration of HCOO- and H+ can be found out.
HCOOH → HCOO− + H+
Initial Concentration 0.019 M 0 0
Equilibrium Concentration (0.019−x) M x x
Where,
- x is the concentration of the ions at equilibrium.
At equilibrium, dissociation constant can be calculated as follows.
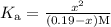
At equilibrium, the concentration of x is negligible as compared to that of HCOOH.
Substitute the value of Ka in the above equation.
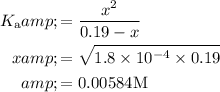
Here, the concentration of hydrogen ion is obtained. From the hydrogen ion concentration, the pH of the solution is found out as follows:
![\begin{aligned}\mathrm{pH} &=-\log \left[\mathrm{H}^(+)\right] \\&=-\log (0.00584 \mathrm{M}) \\&=2.23\end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/college/z30hmklkzuw9j5d4kimp0sfefxktt960yi.png)
Therefore, the pH of 0.19M HCCOH is 2.23